Download Article
See the video: Propagation of Roses by Stenting at Anton Verbeek New Roses International, Holland
It is often necessary to propagate new rose bushes by grafting selected rose cultivars onto rose rootstocks. Seedling rootstocks of Rosa canina, a preferred variety, show genetic variation. To develop a uniform crop these variations are not desired. For the most uniform quality rose rootstocks should be vegetatively propagated by the rooting of cuttings.
STENTING is a method for the quick propagation of roses. Cutting and grafting is performed in one action. In Dutch the word "stenting" means "to stem". It is a combination "stekken" meaning "to strike a cutting" and "enter" meaning "to graft". The success of simultaneous cutting and grafting is cost effective.
In practice, the scion consists of the cultivar stem taken with one leaf and a dormant bud. The scion is grafted on a single internode of the non-rooted rootstock. Formation of the graft union and of adventitious roots on the rootstock occur simultaneously. The combined process takes three weeks.
Background
- Bailey (1896) described the cutting grafting of Morus alba and Morus rubra.
- McFadden (1963) defined a technique to propagate roses by combining cutting and grafting, using Rosa fortuniana as rootstock.
- Van de Pol and Van der Vliet (1979) introduced a technique for Dutch conditions using Rosa chinensis 'Indica Major' as a rootstock and called "stenting".
- Ohkawa (1980) tested cutting grafting of roses under Japanese conditions recommending tongue-grafting of softwood scions on stock cuttings from which buds had been removed.
- Grueber and Hanan (1980), in Israel, described a mini plant system based on side grafting a scion with at least one leaf below the leaf of a rootstock scion. Rooting takes place of the combination.
- Van de Pol and Breukelaar (1981) improved the stenting method by grafting a scion on just one internode without buds onto the rootstock. The result is a lowered problem of wild suckering.
Discussion
The technique of stenting by means of grafting a scion on a rootstock consisting of one bare internode has many advantages.
It is very important that the cultivars are grown on the most suitable rootstock. For cut rose bushes the rootstock must induce vigorous growth. Some rootstocks like the 'Multiflora' types are so vigorous that it is impossible to get a good graft union with some cultivars after budding or bench grafting on an existing root system; the grafted bud or scion is over powered by the activity of the rootstock. Using the stenting process there are no problems because the formation of the graft union and of adventitious roots occur simultaneously.
Stenting is more complicated than the conventional rooting of cuttings. The graft union must be formed before root initiation. After leaves form on the scion there must be a free transport flow of carbohydrates and natural hormones from the leaf to the base of the rootstock. These plant products are used in the rootstock for new root initiation. When a suitable rootstock is used the root formation after stenting can be better than those produced by conventional rooting.
Stenting is a good method to evaluate new scion-stock combinations and to investigate the interaction between shoots and roots.
Stenting can be performed all year. For production purposes stenting provides the possibility of year-around propagation. Growers can utilize expensive grafting equipment more than their normal four months per year besides their traditional propagation techniques.
See a video: stenting done in a commercial greenhouse at Anton Verbeek New Roses International, Holland
General Materials and Methods:
The Rootstock
- The rootstock must be of types which form roots easily after cutting.
- Select mature softwood rootstock material at a stage where the leaves are well developed and the thorns can be broken off easily. By comparison, immature rootstock material is young, soft. and have low survival rates.
- The stem of the rootstock is cut with a sharp pruning knife into pieces consisting of a single internode without buds.
- An internode length of 3/8 inch is sufficient.
- An auxin is applied to the basal part of the rootstock by the quick-dip method.
- Typical rootstock auxin treatments for Rosa chinensis 'Indica Major' use fresh solutions of Hortus IBA Water Soluble Salts r 5000 ppm IBA and for Rosa canina 'Inermis' at 2000 ppm IBA.
The Scion
- The scion cultivar should have the flower just opened with full-grown leaves.
- Typical rose cultivars used in successful trials (Van de Pol, 1980) have been 'Cocktail 80'; 'Dr. Verhage' ('Golden Wave'), 'Hanne', 'Iceberg', 'Motrea', 'Red Success'. 'Sonia' ('Sweet Promise') and 'Sterling Silver'.
The Union

|
The scion is either split grafted by hand with a sharp grafting knife or machine grafted on the internode of the rootstock.
PHOTO:
Left: hand split graft
Right: machine omega graft
|
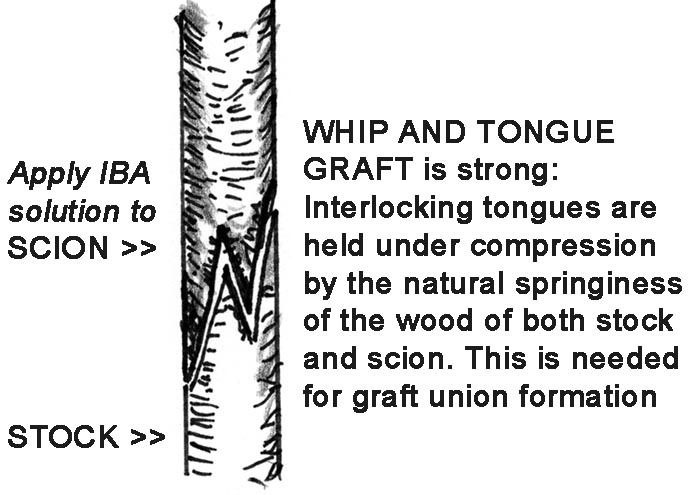
The Technique of Stenting
- The stem of the rose cultivar scion is cut into sections with one leaf with at least five small leaflets and a dormant bud; retain one half inch of stem.
- The scion is either split grafted by hand using a sharp grafting knife or machine grafted on the internode of the rootstock. The machine makes an omega cut in both scion and rootstock to make the parts fit together.
- The grafted parts are tied with cloth or glued paper grafting tape.
- A solution of plant rooting hormones (the auxin) is applied to the basal part of the rootstock by the quick-dip method. Use a fresh solution of Hortus IBA Water Soluble Salts solutions at 5000 ppm IBA for rootstocks like Rosa chinensis 'Indica Major' or 2000 ppm IBA for rootstocks like Rosa canina 'Inermis'.
The Quick Dip Method:
First treat the cuttings then plant them. Immerse the basal end of cuttings approximately one inch in the solution for a few seconds. Plant the cuttings immediately. Use the lowest possible concentration to achieve the desired results. An excess concentration may result in a reduced numbers of roots formed, phyto toxicity, shock, excessive callus, and rooting inequality.
Planting Requirements
- The leaves are moistened to prevent wilting.
- The stentlings are placed in a mixture by volume of one part sand to one part peat
- Media pH of 5.5.
- The plants are planted at about 100 plants per square yard.
- The scion leaves are not overlapped.
- The relative humidity is maintained at about 100%.
- The temperature of medium and atmosphere should be kept at about 75F.
- To prevent premature release of the bud and the stimulation of root growth in dark periods the natural day light must be extended to a full day by lamps. Use supplementary light of least 3000 m W per square yard at plant level.
The Process Sequence
- Grow together the graft union.
- Root formation.
- Growth of the axillary bud.
- After about three weeks the grafted parts will grow together and roots are formed.
- The new stentling can be hardened off and planted out.
Eliminate Wild Suckering
- When the rootstock is initially without buds, suckering can only occur when this rootstock forms adventitious shoots from its newly formed roots.
- Van de Pol (1980) found to diminish wild suckering it is necessary to eliminate buds on the rootstock. Using Rosa chinensis 'Indica Major' as a rootstock the best results were obtained with mature internodes 6- 8 mm in diameter. Use a fresh solution of Hortus IBA Water Soluble Salts at 5000 ppm IBA.
Overcome Special Problems
- During the winter pay extra attention to the degree of ripeness of the plant material. At this time problems like black rot of the rootstock caused by Fusarium oxysporum var. redolens and root formation by the scion may occur. It is preferable to use a lower hormone concentration of a fresh solution of Hortus IBA Water Soluble Salts at 2500 ppm IBA in this period.
- Pretreatment of the rootstocks by cooling can be a way to prevent black rot as well as root formation by the scion.
- Machine grafting may have a labor advantage over hand grafting.
Additional Research
Cummins (1997) states that the 'success of a graft union depends on the establishment of a callus bridge between the cut surfaces of scion and stock and the subsequent establishment of a functioning vascular cylinder connecting scion and stock. Initial callus formation appears to develop about equally on the cut surfaces of both partners, arising not from the cambial layers but from parenchyma cells, mostly in the wood just inside the cambium. Soon after scion and stock calluses have merged, callus cells just below the cambial cells of the scion begin to divide in the same plane as the cambium. Waves of cell division proceed from the top down, suggesting that a regulatory stimulus moves to the cut surface from the growing-shoot tip. Callus parenchyma cells inside the new cambium cylinder re-differentiate into functioning xylem cells. The new cambium begins producing phloem cells.' Upon application of liquid rooting hormones to the graft site, of malus trials, survival was greater for the treated grafts than for those not treated. He used the equivalent of a fresh solution of Hortus IBA Water Soluble Salts at 2000 ppm IBA. Cummins says it is probable that the rooting hormones increase both the formation of a callus and the rate at which the new cambium cylinder is differentiated through the callus parenchyma cells. He suggests that dry powder rooting hormone should also be tried at the graft union.
References
- Bailey, L.H., 1896. The Nursery-Book. MacMillan, London, p. 111.
- Cummins, James, N. Y. State Agricultural Experiment Station, Geneva, NY 14456. Pomona, Spring 1997, v. 2, no. 34 XXX.
- Garner, R.J., 1958. The Grafters Handbook. Faber and Faber, London, p. 171.
- Grueben, K.L. and Hanan, J.J., 1980. Rose rootstocks and mini plant propagation: preliminary results and observations. Colo. Greenhouse Growers Assoc., 364: 1 2.
- McFadden, S.E., 1963. Grafting leafy stem cuttings, a technique for propagating roses. Proc. Florida State Horticultural. Soc., 76: 412 416.
- Ohkawa, K., 1980. Cutting-grafts as a means to propagate greenhouse roses. Scientia Horticulturae, 13: 191 199.
- Pol, P.A. van de, and Vliet, G. van der, 1979. Rozen stekken en enten in een handeling. Vakbl. Bloemisterij, 26: 40 41.
- Pol, P.A. van de, and A. Breukelaar, Agricultural University, Department of Horticulture, Wageningen (The Netherlands). 1982. Scientia Horticulturae (1982), 17: 187-196.
Stenting Roses- Effects of IBA application and the types of cuttings (2015)
Propagation of Roses
Article: Stenting: Rooting of Cuttings and Grafting at the Same Time
Article Download |